#534 What mass of KIO3 is needed to convert the copper
What mass of KIO3 is needed to convert the copper - Chemistry
ChemistryExplain daily providing Q&A content “#534 What mass of KIO3 is needed to convert the copper" in Chemistry, ACS organic chemistry study guide, Adhesion chemistry, Aleks chemistry, Analytical chemistry jobs, Aromatic Chemistry, Carbon chemistry
Get the Free Online Chemistry Q&A Questions And Answers with explain. To crack any examinations and Interview tests these Chemistry Questions And Answers are very useful. Here we have uploaded the Free Online Chemistry Questions. Here we are also given the all chemistry topic.
ChemistryExplain team has covered all Topics related to inorganic, organic, physical chemistry, and others So, Prepare these Chemistry Questions and Answers with Explanation Pdf.
For More Chegg Questions
Join Our Telegram Channel for Covers All Update by ChemistryExplain:- Click Now
Free Chegg Question
What mass of KIO3 is needed to convert the copper in 0.2750 g of CuSO4 • 5H2O to Cu(IO3)2?
Free Chegg AnswerFor More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
Free Chegg Answer
-
Step 1 of 2
First, write out the chemical equation.
-
Step 2 of 2
Use the stoichiometric coefficients and molar masses to perform unit analysis and determine the mass of KIO3 needed for the given amount of CuSO4 pentahydrate.
To convert CuSO4 pentahydrate to Cu(IO3)2, 0.4714 g KIO3 is required.
Labels: Chegg, Free Chegg Answer, Q&A Chemistry

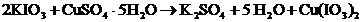
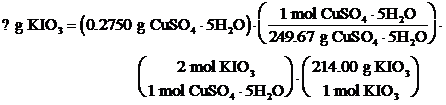


0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home