#498 A piston-cylinder device contains 2.2 kg of nitrogen
A piston-cylinder device contains 2.2 kg of nitrogen - Mechanical Engineering
ChemistryExplain daily providing Q&A content “#498 A piston-cylinder device contains 2.2 kg of nitrogen" in Mechanical Engineering, Best colleges for mechanical engineering, Entry level mechanical engineer, Mechanical engineering companies
Get the Free Online Chemistry Q&A Questions And Answers with explain. To crack any examinations and Interview tests these Chemistry Questions And Answers are very useful. Here we have uploaded the Free Online Chemistry Questions. Here we are also given the all chemistry topic.
ChemistryExplain team has covered all Topics related to inorganic, organic, physical chemistry, and others So, Prepare these Chemistry Questions and Answers with Explanation Pdf.
For More Chegg Questions
Join Our Telegram Channel for Covers All Update by ChemistryExplain:- Click Now
Free Chegg Question
A piston-cylinder device contains 2.2 kg of nitrogen initially at 100 kPa and 25°C. The nitrogen is now compressed slowly in a polytropic process during which 
Free Chegg AnswerFor More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
Free Chegg Answer
-
Step 1 of 7
Express the energy balance equation for closed system.
Here, energy transfer in to the system is
, energy transfer from the system is
and change in internal energy of system is
.
Substitute
for
,
for
and
for .
……. (1)
Here, boundary work done input is
, amount of heat transfer exit from the system is
and change in internal energy is
.
Substitute
for
in Equation (1).
……. (2)
Here, mass of system is m, specific heat at constant volume is
, initial temperature is
and final temperature is
.
-
Step 2 of 7
Calculate the final pressure
of nitrogen gas inside the cylinder.
Here, initial pressure is
and initial volume is
and final volume is
.
Consider the volume is reduced by one half due to compress of nitrogen gas in polytrophic process.
Substitute, 2 for
and
for
.
-
Step 3 of 7
Calculate the final temperature
of oxygen in the tank.
Here, initial temperature is
, final temperature is
and initial pressure is
.
Convert the unit of initial temperature from degree Celsius to Kelvin.
Substitute
for
,
for
, 2 for
and
for
.
-
Step 4 of 7
Express the boundary work done input for polytrophic process.
Here, pressure inside the cylinder is P.
Integrate the above equation.
……. (3)
Here, the polytrophic constant is n, the initial volume of piston-cylinder is
, the final volume of piston-cylinder is
, the initial pressure inside the cylinder is
and final pressure inside the cylinder is
.
-
Step 5 of 7
Substitute
for
and
for
in Equation (3).
Here, the mass of nitrogen gas inside the cylinder is m, the gas constant is R, initial temperature is
and final temperature is
.
From the gas constant properties table, select the gas constant of nitrogen as
.
-
Step 6 of 7
Substitute 1.3 for n,for R,
for m,
for
and for
.
Hence, the boundary work done input for the polytrophic process is
.
-
Step 7 of 7
Calculate the amount of heat transfer
exit from the cylinder.
From the ideal gas specific heats of various common gases table, select the specific heat at constant volume of nitrogen at temperature of 300 K as
.
Substitute
for m,
for
,
for ,
for
and
for
.
Hence, the amount of heat transfer exit from the cylinder is
.
Labels: Chegg, Free Chegg Answer, Q&A Mechanical Engineering








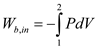







0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home