#164 How many chiral centers are present in each of the
How many chiral centers are present in each of the - Organic Chemistry
ChemistryExplain daily providing Q&A content “#164 How many chiral centers are present in each of the" in Organic Chemistry, Organic chemistry online course, Acs organic chemistry, Organic chemistry jobs.Get the Free Online Chemistry Q&A Questions And Answers with explain. To crack any examinations and Interview tests these Chemistry Questions And Answers are very useful. Here we have uploaded the Free Online Chemistry Questions. Here we are also given the all chemistry topic.
ChemistryExplain team has covered all Topics related to inorganic, organic, physical chemistry, and others So, Prepare these Chemistry Questions and Answers with Explanation Pdf.
For More Chegg Questions
Free Chegg Question
How many chiral centers are present in each of the following molecular structures?a.For More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
b.
c.
d.
Free Chegg Answer
The chiral center is the point or carbon atom which possesses all the four different groups attached to it. A chiral center is commonly denoted by an asterisk (*).
(a)
The structure of the given molecule is as follows:
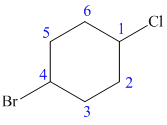
Now we can find the carbon atom is chiral or not as described as follows:
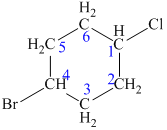
Carbon number 1 and 4 consists of one Cl (chlorine) atom and one H (hydrogen) atom attached to them. But both these carbons consist of the same groups (CH2-CH2-CH-Br) at their left and right side. Thus, C-1 and C-4 carbon atoms are not chiral centers.
Carbon number 2, 3, 5, and 6 consists of two same groups (hydrogen atoms). From the definition, it gets clear that a chiral carbon is the one that has all the four different groups attached to it. No two groups can be similar. Thus, C-2, C-3, C-5, and C-6 carbon are not chiral centers.
Hence, the given structure does not show any chiral centers.
(a)
The structure of the given molecule is as follows:

Now we can find the carbon atom is chiral or not as described as follows:
Carbon number 1 and 4 consists of one Cl (chlorine) atom and one H (hydrogen) atom attached to them. But both these carbons consist of the same groups (CH2-CH2-CH-Br) at their left and right side. Thus, C-1 and C-4 carbon atoms are not chiral centers.
Carbon number 2, 3, 5, and 6 consists of two same groups (hydrogen atoms). From the definition, it gets clear that a chiral carbon is the one that has all the four different groups attached to it. No two groups can be similar. Thus, C-2, C-3, C-5, and C-6 carbon are not chiral centers.
Hence, the given structure does not show any chiral centers.
(b)
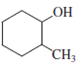
The structure of the given molecule is as follows:
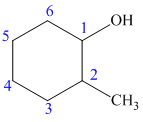
Now we can find the carbon atom is chiral or not as described as follows:
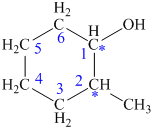
Carbon number 1 consists of four different groups attached to it. It consists of one hydrogen atom, one hydroxyl (OH) group, CH2-CH2-CH2 (propyl) group on its one side and CHCH3-CH2-CH2 (1-methyl propyl) group on its other side. Thus, all the four groups are different, thus, the C-1 carbon is a chiral center.
Carbon number 2 consists of four different groups attached to it. It consists of one hydrogen atom, one methyl group, CH2-CH2-CH2 (propyl) group on one side and CHOH-CH2-CH2 (1-hydroxy propyl) group on the other side. Thus, all the four groups are different, thus, the C-2 carbon is a chiral center.
Hence, the given structure shows two chiral centers.
The structure of the given molecule is as follows:

Now we can find the carbon atom is chiral or not as described as follows:
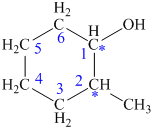
Carbon number 1 consists of four different groups attached to it. It consists of one hydrogen atom, one hydroxyl (OH) group, CH2-CH2-CH2 (propyl) group on its one side and CHCH3-CH2-CH2 (1-methyl propyl) group on its other side. Thus, all the four groups are different, thus, the C-1 carbon is a chiral center.
Carbon number 2 consists of four different groups attached to it. It consists of one hydrogen atom, one methyl group, CH2-CH2-CH2 (propyl) group on one side and CHOH-CH2-CH2 (1-hydroxy propyl) group on the other side. Thus, all the four groups are different, thus, the C-2 carbon is a chiral center.
Hence, the given structure shows two chiral centers.
(c)
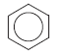
The structure of the given molecule is as follows:
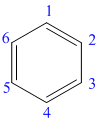
Now we can find the carbon atom is chiral or not as described as follows:
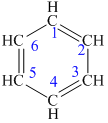
All the carbon atoms consist of double bonds. According to the definition, a chiral center should have four different groups attached to it. Since the double bonded carbon atoms consists of only three groups, thus, the structure has no chiral center. Thus, all the carbons are not chiral centers irrespective of any substituent attached to it.
Hence, the given structure does not show any chiral centers.
The structure of the given molecule is as follows:

Now we can find the carbon atom is chiral or not as described as follows:
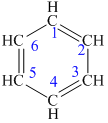
All the carbon atoms consist of double bonds. According to the definition, a chiral center should have four different groups attached to it. Since the double bonded carbon atoms consists of only three groups, thus, the structure has no chiral center. Thus, all the carbons are not chiral centers irrespective of any substituent attached to it.
Hence, the given structure does not show any chiral centers.
(d)
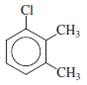
The structure of the given molecule is as follows:
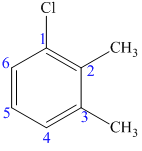
Now we can find the carbon atom is chiral or not as described as follows:
All the carbon atoms consist of double bonds. According to the definition, a chiral center should have four different groups attached to it. Since the double-bonded carbon atoms consist of only three groups, thus, the structure has no chiral center. Thus, all the carbons are not chiral centers irrespective of any substituent attached to it.
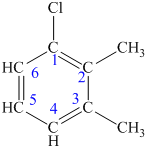
The structure of the given molecule is as follows:

Now we can find the carbon atom is chiral or not as described as follows:

All the carbon atoms consist of double bonds. According to the definition, a chiral center should have four different groups attached to it. Since the double-bonded carbon atoms consist of only three groups, thus, the structure has no chiral center. Thus, all the carbons are not chiral centers irrespective of any substituent attached to it.
Labels: Chegg, Free Chegg Answer, Organic Chemistry


0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home