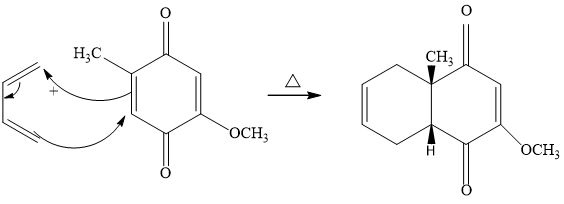
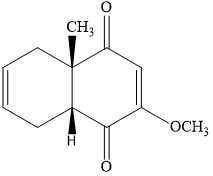
#281 How many hydrogens are bonded to each
How many hydrogens are bonded to each - Organic Chemistry
ChemistryExplain daily providing Q&A content “#281 How many hydrogens are bonded to each" in Organic Chemistry, Organic chemistry online course, Acs organic chemistry, Organic chemistry jobs
Get the Free Online Chemistry Q&A Questions And Answers with explain. To crack any examinations and Interview tests these Chemistry Questions And Answers are very useful. Here we have uploaded the Free Online Chemistry Questions. Here we are also given the all chemistry topic.
ChemistryExplain team has covered all Topics related to inorganic, organic, physical chemistry, and others So, Prepare these Chemistry Questions and Answers with Explanation Pdf.
For More Chegg Questions
Free Chegg Question
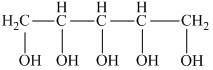
In the compound below
a) How many hydrogens are bonded to each carbon in the following compounds?
b) What is the molecular formula?
For More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
Free Chegg Answer
- Each carbon atom of this compound is a different-different number of hydrogen-bonded.
- Molecular formula - for molecular formula count the number of atoms with
different type - so the molecular formula is - C10H14O4
Labels: Chegg, Free Chegg Answer, Q&A Organic Chemistry










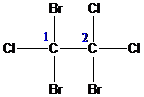
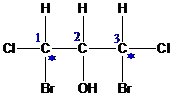







 .
. .
.




















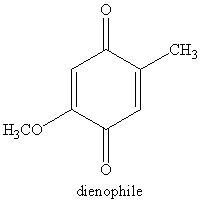
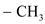 group containing dienophile react more readily than
group containing dienophile react more readily than 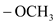 group containing dienophile, because,
group containing dienophile, because, 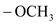 group is good electron donating group than
group is good electron donating group than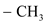 group.
group.