Radial And Angular Functions
Radial And Angular Functions - Atomic structure and the periodic table
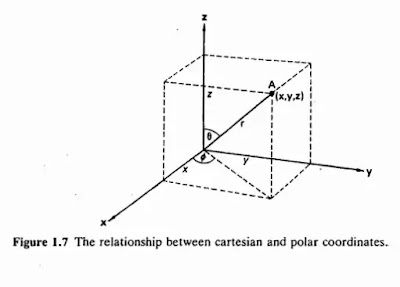
Chemistry Explain provide notes about Atomic structure and the periodic table today we discuss “Radial And Angular Functions” Inorganic ChemistryThe Schrodinger equation can be solved completely for the hydrogen atom, and for related ions which have only one electron such as He+ and u2+. For other atoms only approx: create solutions can be obtained. For most calculations, it is simpler to solve the wave equation if the cartesian coordinates x, y, and z are converted into polar coordinates r, e, and. The coordinates of the point A measured from the origin are x, y, a.rid z in cartesian coordinates, and r, e, and in polar coordinates. It c·an be seen that the two sets of coordinates are related by the following expressions:
R(r) is a function that depends on the distance from the nucleus, which in turn depends on the quantum numbers n and I
Θ(θ) is a function of 0; which depends on the quantum numbers I and m Φ(Φ) is a function of cp, which depends only on the quantum number m
Equation (1.6) may be rewritten
This splits the wave function into two. pa~ts which can be solved separately: 1. R(r) the radial function, which depends on the· quantum numbers n and/.
2. Aml the total angular wave function, which depends on the quantum numbers m and I.
The radial function R has no physical meaning, but R2 gives the probability of finding the electron in a small volume DV near the point at which R is measured. For a given value of r the number of small volumes is 4πr2, so the probability of the electron being at a distance. r from ·the nucleus is 4πr2R2• This is called the radial distribution function. Graphs of the
the radial distribution function for hydrogen plotted against r is shown in Figure 1.8.
For More Chegg QuestionsThese diagrams show that the probability is zero at the nucleus (as r = 0), and by examining the plots for ls, 2s and 3s that the most probable distance increases markedly as the principal quantum number increases. Furthermore, by comparing the plots for 2s and 2p, or 3s, 3p, and 3d it can be seen that the most probable radius decreases slightly as the subsidi_ary quantum number increases. All the~· orbitals except the first one (ls) have a shell-like structure, rather like an onion or a hailstone, consisting of concentric layers of electron density. Similarly, all bun he first p orbitals (2p) and the first d orbitals (3d) have a shell structure.
The angular function A depends only on the direction and is independent of the distance from the nucleus (r). Thus A2 is the probability of
finding an electron at a given direction θ, Φ at any distance from the nucleus to infinity. The angular functions Aare plotted as polar diagrams in Figure 1.9. It must be emphasized that these polar diagrams do not represent the total wave function u, but only the angular part of the wave function. (The total wave function is made up of contributions from both the radial and the angular functions.)
Thus the probability of finding an electron simultaneously at a distance r and in a given direction θ, Φ is Ψ2rθ, Φ
Polar diagrams, that are drawings of the angular part of the wave function, are commonly used to illustrate the overlap of orbitals giving bonding between atoms. Polar diagrams are quite good for this purpose, as they show the signs + and - relating to the symmetry of the angular function. For bonding like signs must overlap. These shapes are slightly different from the shapes of the total wave function. There are several points about such diagrams:
l. It is difficult to picture an angular wave function as a mathematical equation. It is much easier to visualize a boundary surface, which is a solid shape. which for example contains 90% of the electron density. To emphasize that Ψ is continuous. function, the boundary surfaces have been extended up to the nucleus in Figure 1.9. For p orbitals, the electron density is zero at the nucleus, and stone texts show a p orbital a' two spheres which do not touch.
2. These drawings show the symmetry for the ls, 2p, 3d orbitals. However, in the others, 2s, 3s, 4s . .. , 3p, 4p, Sp . .. , 4d, Sd . .. the sign (symmetry) changes inside the boundary surface Of the orbital. this is readily seen as nodes in the graphs Of the radial functions (Figure 1.8).
For More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
3. The probability of finding an electron at a direction θ, Φ is the wave function squared, A2 , or more precisely Ψ2θ, Ψ2ΦThe diagrams in Figure 1. 9 are of the angular part of the wave function A, not A2 Squaring does not change the shape of an s orbital, but it elongates the lobes of p orbitals (Figure 1.10). Some books use elongated p orbitals, but strictly these should not have signs, as squaring removes· any sign from the symmetry. Despite this, many authors draw shapes approximating to the probabilities, i.e. squared wave functions, and put the signs of the wave function on the lobes, and refer to both the shapes and the wave functions as orbitals.
4. A full representation of the probability of finding an electron requires the total wave function squared and includes both the radial and angular probabilities squared. It really needs a three-dimensional model to display this probability and show the shapes of the orbitals. It is difficult to do this adequately on a two-dimensional piece of paper, but a representation is shown in Figure 1.11. The orbitals are not drawn to scale. Note that the p orbitals are not simply two spheres, but are ellipsoids of revolution. Thus the 2px orbital is spherically symmetrical about the x axis, but is not spherical in the other direction. Similarly, the Py orbital is spherically symmetrical about the y axis, and both the Pz and the 3dz2 are spherically symmetrical about the z axis.
Labels: Atom, Atomic structure, Bohr Theory, Inorganic Chemistry












0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home