#212 When spring finally arrives in the mountains
When spring finally arrives in the mountains - Physics
ChemistryExplain daily providing Q&A content “#212 When spring finally arrives in the mountains" in Physics, Ap physics 1 practice test, Best colleges for physics, Best physics books, Best physics schools
Get the Free Online Chemistry Q&A Questions And Answers with explain. To crack any examinations and Interview tests these Chemistry Questions And Answers are very useful. Here we have uploaded the Free Online Chemistry Questions. Here we are also given the all chemistry topic.
ChemistryExplain team has covered all Topics related to inorganic, organic, physical chemistry, and others So, Prepare these Chemistry Questions and Answers with Explanation Pdf.
For More Chegg Questions
Free Chegg Question
When spring finally arrives in the mountains, the snow pack may be two meters deep, composed of 50% ice and 50% air. Direct sunlight provides about. 1000 watts/m2 to earth’s surface, but the snow might reflect 90% of this energy. Estimate how many weeks the snow pack should last, if direct solar radiation is the only source of energy.
For More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - Chemistry Explain
Free Chegg Answer
During fusion of ice, the heat released by water and absorbed by the air is given by the following formula;
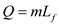
Here, m is the mass of ice and  is the latent heat of fusion of ice.
is the latent heat of fusion of ice.
The snow will melt because of the heat energy coming from the sunlight.
Since 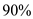 of energy reflects back hence only
of energy reflects back hence only 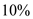 of energy is only being used to melt the snow.
of energy is only being used to melt the snow.
Then, the amount of heat generated by the sunlight is used in melting of snow can be calculated as,

The depth of the snow pack would be,
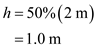
The time for which the snow pack will exist would be,
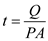
Here, 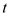 is the time,
is the time, 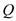 is the rate of heat transfer,
is the rate of heat transfer, 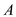 is the area of heat transfer and
is the area of heat transfer and 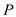 is the solar energy flux
is the solar energy flux
The amount of heat transfer during the melting of snow per unit area can be calculated as follows,

Here, 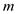 is the mass of the snow pack,
is the mass of the snow pack, 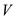 is the volume of the snow pack,
is the volume of the snow pack, 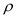 is the density of the ice,
is the density of the ice, 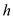 is the depth of the snow pack and
is the depth of the snow pack and  is the latent heat of fusion of ice.
is the latent heat of fusion of ice.
So, the time can also be written as follows,
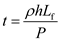
Substitute,  for
for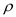 ,
, 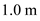 for
for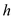 ,
, 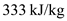 for
for  and
and 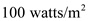 for
for 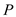 in the above expression to get the time required as follows,
in the above expression to get the time required as follows,

Convert the required time from seconds to week.
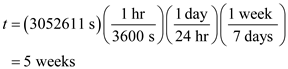
Thus, 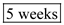 the snow pack will last.
the snow pack will last.
Labels: Chegg, Free Chegg Answer, Q&A Physics


0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home