(Localized Chemical Bonding) Covalency of Carbon, Orbital (Wave Mechanical) Concept of Bonding, Atomic Orbitals - Structure and Bonding
ChemistryExplain provide notes about Localized Chemical Bonding today we discuss “Localized
Chemical Bonding - Covalency of Carbon, Orbital Concept of Bonding, Atomic Orbitals”
Organic Chemistry, Organic chemistry online course, Acs organic chemistry, Organic chemistry jobs
Covalency of Carbon
Carbon has four valency electrons and usually, it acquires the stable electronic configuration of the nearest noble gas neon by sharing its electrons with other atoms. Thus, carbon is tetracovalent (quadrivalent). Lewis and Couper structures of some covalent carbon compounds are given below :
Carbon atoms have the unique ability to join together to form very long chains and large rings, which can have branches and cross-links. This unique property of carbon is known as catenation, which is responsible for the wide variety and a large number of carbon compounds. Since catenation is not possible to such an extent for atoms of any other element, inorganic compounds are far less in number.
For More Chegg Questions
Orbital (Wave Mechanical) Concept of Bonding
The electronic theory of bonding could not answer fundamental questions concerning, e.g., the bond energy, and geometry and shapes of molecules. The emergence of quantum (wave) mechanics ultimately led to the development of two theories of bonding, viz., the valence bond (VB) theory and the molecular orbital (MO) theory to explain the nature of covalent bonds. Both of these theories involve atomic orbitals (AO's), thus before intelligently discussing them, we must consider the shapes and orientations of atomic orbitals.
For More Chemistry Notes and Helpful Content Subscribe Our YouTube Chanel - ChemistryExplain
Atomic Orbitals
An atomic orbital is defined as the definite region in three-dimensional space around a nucleus where there is a high probability of finding an electron of specific energy. In organic chemistry, we are mainly concerned with sand p-orbitals, hence the shapes and orientations of these orbitals are described below:
An s orbital has a spherical shape. The atomic nucleus in the center of\\S orbital and the orbital is spherically symmetrical about the nucleus.
A p atomic orbital consists of two equal lobes forming a dumb-bell shape. The two lobes do not touch each other at the nucleus, thus the probability of finding electrons in this region is zero and it is called the nodal plane. There are three p orbitals of equal energy. They are directed perpendicular to each other just as Cartesian co-ordinates and are designated as P
x, P
y, and P
z corresponding to' their axes of orientation.
Solid State
If the forces of attraction between molecules are much greater than the thermal energy, the positions of the molecules remain fixed and we have matter in the solid-state. The molecules in the solid-state, therefore, do not possess any translational energy but have only vibrational energy since they can vibrate about their mean positions. Extremely large forces of attraction exist amongst them. That is way solids differ markedly from liquids and gases in respect of size, shape volume. Solids, in general, have definite size, shape, and volume.:
Labels: Localized Chemical Bonding, Organic Chemistry, Organic Chemistry Notes





























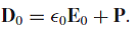

 is the dielectric field.
is the dielectric field.

 is the charge is enclosed on the surface and is the small area.
is the charge is enclosed on the surface and is the small area.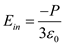 â¦â¦ (1)
â¦â¦ (1) is permittivity in free space.
is permittivity in free space. is the electric field inside the dielectric material, then total field at the center inside the cavity is
is the electric field inside the dielectric material, then total field at the center inside the cavity is  â¦â¦ (2)
â¦â¦ (2) â¦.. (3)
â¦.. (3)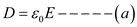

 â¦â¦ (4)
â¦â¦ (4)
 in equation (4),
in equation (4),
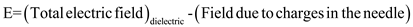
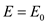 .
.
 â¦â¦ (5)
â¦â¦ (5) .
.


 â¦â¦ (6)
â¦â¦ (6) .
.
 â¦â¦ (7)
â¦â¦ (7)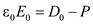
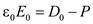 in equation (7),
in equation (7),
